

If something is impossible, you have to accept it and find a way around it. That makes it impossible to plot an orbit for an electron around a nucleus.
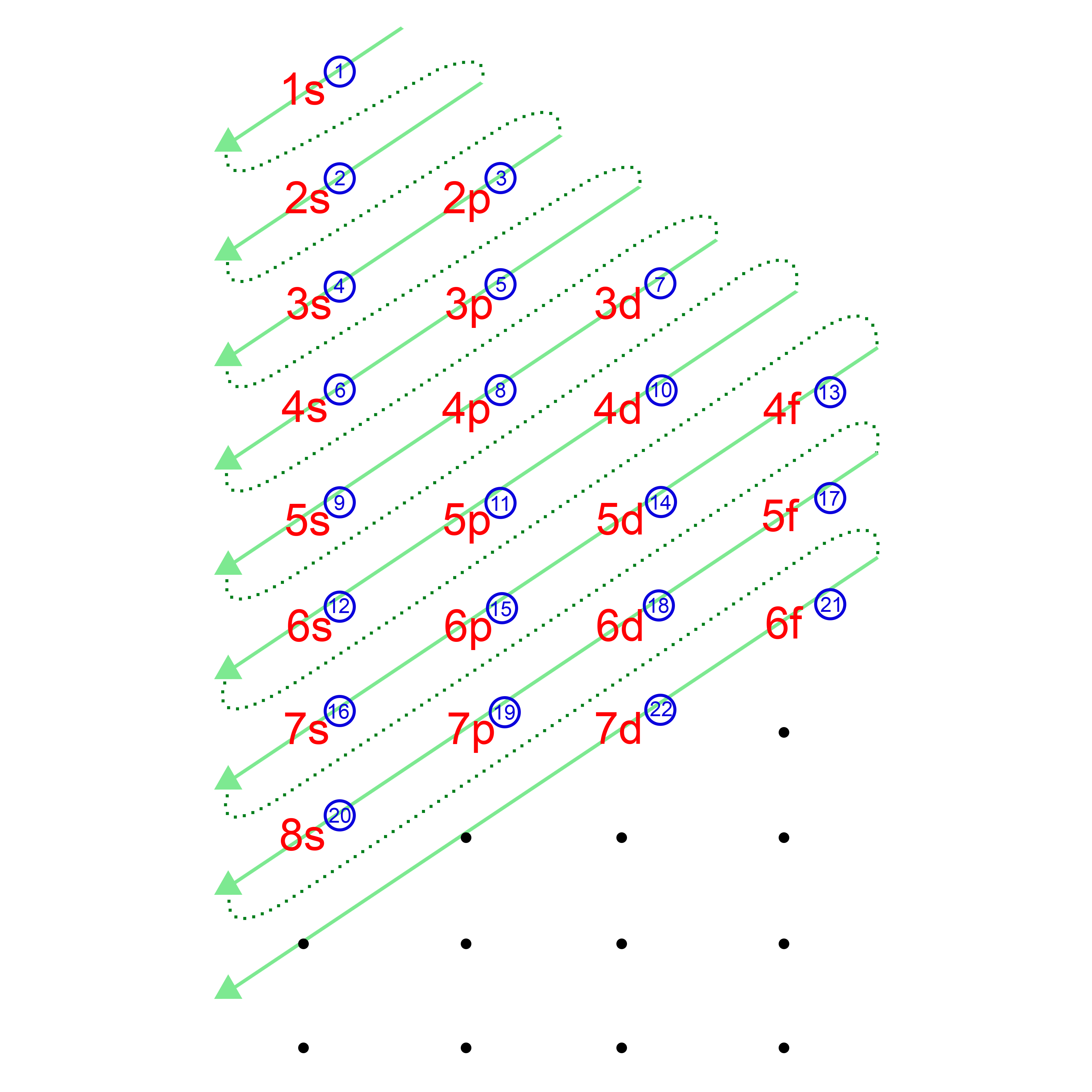
(What it actually says is that it is impossible to define with absolute precision, at the same time, both the position and the momentum of an electron.) The Heisenberg Uncertainty Principle says - loosely - that you can't know with certainty both where an electron is and where it's going next. To plot a path for something you need to know exactly where the object is and be able to work out exactly where it's going to be an instant later. The impossibility of drawing orbits for electrons It is essential that you understand the difference between them. Orbits and orbitals sound similar, but they have quite different meanings. The truth is different, and electrons in fact inhabit regions of space known as orbitals. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. d orbitals are described only in terms of their energy, and f orbitals only get a passing mention. It explores s and p orbitals in some detail, including their shapes and energies.
This page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as UK A level and its equivalents.


 0 kommentar(er)
0 kommentar(er)
